Modelling Aerosol Composition
Atmospheric aerosols are very small (up to 0.1 microns) particles that are present in the air all around us. They are important because they both scatter and absorb sunlight and influence the formation of clouds, (and thus have an impact upon global climate); and also they can be inhaled into the deepest recesses of the lungs with implications for health. They can be solid, liquid, or a mixture of both and can contain a wide range of components, but most are believed to be mainly composed of an inorganic part, an organic part and associated water. The inorganic component is relatively well understood and is composed of a limited number of ionic species. However, the organic component is much more complex as any of the thousands of organic compounds found in the atmosphere can potentially condense into the aerosol. Volatile organic compounds (VOC’s) from both man-made (anthropogenic- eg. vehicle engine exhaust) and natural sources (biogenic- eg. terpenes released from trees and plants) undergo progressive oxidation in the atmosphere and the products may contribute to the formation of new organic aerosol (OA) particles. The oxidation process leads to increased functionalization of the VOC with a resulting increase in polarity and decrease in volatility.
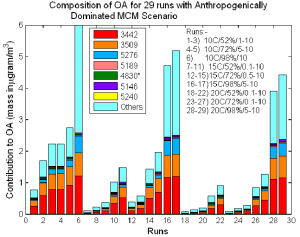
There is much speculation about the composition of OA particles as complete analyses are not available. One approach is to model the formation of OA using an explicit atmospheric chemistry scheme coupled to a condensation/absorption model. We have used the Master Chemical Mechanism (MCM:- see http://mcm.leeds.ac.uk/MCM/) with a modified version of the Pankow absorption model (Pankow 1994, Barley et al., 2009) to predict the chemical composition of OA particles formed under conditions of varying temperature and relative humidity. (see Figure). A key input to the partitioning model is the vapour pressure of each compound. It has recently been shown that a range of vapour pressure estimation methods give significantly divergent results for the vapour pressures of 45 multifunctional compounds and that these differences have a substantial impact upon the amount of OA predicted by the partitioning model (Barley & McFiggans 2010). We plan to investigate how changing the vapour pressure estimation model influences the composition of the predicted OA.
References
Pankow, J. F. (1994). Atmos. Environ., 28, 185-188.
Barley, M. H., Topping, D. O., Jenkin, M. E. and McFiggans, G. (2009). Atmos. Chem. Phys., 9, 2919-2932.
Barley, M. H., & McFiggans, G. (2010). Atmos. Chem. Phys., 10, 749-767.