Investigations of Aerosol Surface Tension
A lack of fundamental data on pure organic aerosol component properties, including the effects of coupling with inorganic components, causes significant uncertainties in the ability to capture aerosol evolution and in turn the associated impacts on the environment. Important uncertainties are in those parameters which dictate the aerosol water content which in turn is necessary for predicting the direct and indirect climatic effect; The particle’s equilibrium composition and the vapour pressure of each component above the aerosol particle are governed by two effects, the Raoult and Kelvin effects. The Raoult effect considers the influence of interactions taking place in solution on the bulk equilibrium composition and equilibrium partial pressure of components above a solution. Prediction of the equilibrium composition of multicomponent particles requires calculations of component ‘activity’ which is a thermodynamic quantity representing an ‘effective’ concentration; be it of water or any solute. In addition to this, the vapour pressures of all volatile components are higher over a curved surface than a flat one. The Kelvin effect accounts for this enhancement as a result of curvature by reference to the surface tension of the multicomponent solution. With respect to water content, the solution surface tension becomes very important at the point at which the aerosol particle activates into a cloud droplet. At this point the importance of accurate calculations of water ‘activity’ and multicomponent surface tensions switches, the latter more important for cloud activation predictions (indirect effect) and more dilute solutions, the former for growth below 100%RH (direct effect) and more concentrated solutions. These two effects are represented in the Kohler equation describing the equilibrium conditions for water over a solution droplet:
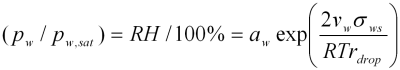
The exponential term represents the Kelvin effect where vw is the partial molar volume of water, σws is the solution surface tension, R is the universal gas constant, T the temperature and rdrop the droplet radius. aw is the water activity (or effective water content on a mole fraction scale) which represents the Raoult effect. Also,pw is the partial pressure of water vapour at ambient conditions, pw,sat the saturation vapour pressure of water vapour and RH is the ambient relative humidity. For a flat surface or particle size for which the exponential term is negligible (~100nm radius), the equilibrium water activity is equal to the ambient saturation ratio of water vapour, i.e. relative humidity on a scale of 0 to 1. For a smaller particle, or system for which the Kelvin term is not negligible (roughly less than 100nm radius), the water activity, or effective concentration of water, is less than that of a flat surface for a given RH.
Most applications of Kohler theory has in the past assumed that the total solute concentrations define both the water activity and surface tension. However, it has been postulated that bulk to surface partitioning, in which molecules from the bulk phase are removed to a surface phase, affect concentrations used to calculate both the Raoult and Kelvin terms. The impact of bulk to surface partitioning has been investigated in various studies. The result of which is a predicted increase in the critical saturation at which an aerosol particle activates into a cloud droplet. From equation (1), solution surface tension is required for cloud activation predictions. This is still true even when one considers bulk to surface partitioning, as a knowledge of the surface tension isotherm is crucial for predicting how much material partitions between a bulk and surface phase. Further work is required to investigate this phenomena before any simplifications can be drawn. Specifically, assumptions often used to reduce equation (1) to a more usuable form require further experimental validation. Surface tension of mixtures of organic solutions and solutions of mixed inorganic / organic compounds have received relatively little attention. Reconciliation of the predictions with ambient measurements of surface tension has been difficult to achieve. This is largely cuased by an inability to identify chemical components associated with the organic fraction. Recent investigations have shown that predictive models perform poorly at capturing both binary organic and mixed inorganic/organic surface tensions and that experimental data is crucial for modelling multicomponent systems; for example, if the influence of organic compounds is neglected in multi-component aerosols this can lead to an over prediction of the required super-saturation of water vapour for cloud activation by up to 80%. Similarly use of erroneous predictive models which attempt to capture the effect of organics can lead to an under-prediction by two orders of magnitude.
References
Booth, A.M., Topping, D.O., McFiggans, G. and Percival, C.J. (2009) Design Surface tension of mixed inorganics and dicarboxylic acid aqueous solutions at 298.15 K and their importance for cloud activation predictions, PCCP, 11(36), 8021-8028.