Vapour Pressure Measurements
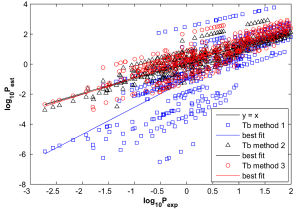
Knowledge of pure component vapour pressures is essential for calculations of gas/particle partitioning of compounds forming atmospheric aerosols. There are many methods of estimating vapour pressures but most of the experimental data collected to date has been for intermediate or high pressure compounds (and often measured at temperatures considerably above ambient) and the proportion of experimental data for low (less than 100 Pa) vapour pressure compounds has been very small. Hence the datasets used for developing the estimation methods have reflected this bias in addition to the fact that components studied tend to have one or two functional groups at the most. The most intensively studied group are hydrocarbons for the oil industry with no functional groups. However, the vapour pressure of simple hydrocarbons are of less interest to the atmospheric community who are often concerned with multi-functional and heavily oxygenated hydrocarbons (Johnson et al., 2006; Yu et al., 1999). Therefore it is unsurprising that some of the estimation methods can give errors in vapour pressure of several orders of magnitude for multifunctional compounds at ambient temperatures. The vapour pressure of all aerosol components are necessary to calculate the mass flux of condensing and evaporating compounds. Pure component vapour pressure data are lacking for the majority of multifunctional organic condensable compounds predicted to be formed from gas phase volatile organic carbon (VOC) oxidation. Furthermore, the available predictive techniques for vapour pressures are largely unevaluated on multifunctional compounds. It is therefore necessary to evaluate such predictive techniques for selection of those most appropriate for atmospheric application and this requires a reliable method of determining vapour pressures of low volatility compounds at ambient temperatures.
We use Knudsen Effusion Mass Spectrometry (KEMS) to measure solid state vapour pressures and Differential Scanning Calorimetry (DSC) to obtain the thermochemical data to get sub-cooled liquid vapour pressures. The experimental work is driven by Dr Murray Booth and the theory by Dr Mark Barley.
Experimental Data
Download experimental vapour pressure data for 45 multifunctional compounds (Excel Spreadsheet 67Kb)
If you use this data please quote the URL where you found it and reference the Barley and McFiggans paper below.
This work was carried out within the UK NERC-funded "Quantifying the Earth SysTem" (QUEST) project under the "QUest Aerosol and Atmospheric Chemistry" (QUAAC) grant number NE/C001613/1; EU-funded "European Integrated project on Aerosol Cloud Climate and Air Quality interactions" (EUCAARI) under contract number 036833-2, and the ACCENT program on Joint Research of Aerosols; Air Quality and Climate
References
M. H. Barley and G. McFiggans: The critical assessment of vapour pressure estimation methods for use in modelling the formation of atmospheric organic aerosol Atmos. Chem. Phys., 10, 749-767, 2010
Booth, A. M., Barley, M. H., Topping, D. O., McFiggans, G., and Garforth, A.: Solid state and sub-cooled liquid vapour pressures of substituted dicarboxylic acids using Knudsen Effusion Mass Spectrometry (KEMS) and Differential Scanning Calorimetry, Atmos. Chem. Phys. Discuss., 10, 5717-5749, 2010