Knudsen Effusion Mass Spectrometer
Knowledge of pure component vapour pressures is essential for calculations of gas/particle partitioning of compounds forming atmospheric aerosols. There are many methods of estimating vapour pressures but most of the experimental data collected to date has been for intermediate or high pressure compounds (and often measured at temperatures considerably above ambient) and the proportion of experimental data for low (less than 100 Pa) vapour pressure compounds has been very small. KEMS is an established vapour pressure measurement technique capable of measuring vapour pressures from 101-10-8 Pa. Secondary organic aerosol (SOA) components are likely to have vapour pressures around 10-4 Pa, measurable at ambient temperature, well inside the range measurable by KEMS.
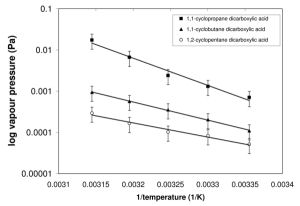
A KEMS system has been built at Manchester by Dr Murray Booth. It consists of temperature controlled Knudsen effusion cell, suitable for controlled generation of a molecular beam of the sample organic compounds in a vacuum chamber, coupled to a quadrupole mass spectrometer. The system is calibrated using the mass spectrometer signal from a sample of known vapour pressure, a load-lock allows the ioniser filament to be left on, then a new sample of unknown vapour pressure can be measured. A Clasius-Clayperon plot of the date (see figure 1) gives us the enthalpy and entropy of sublimation.
Solid state vapour pressures measured in the KEMS can then be converted to sub-cooled liquid vapour pressures using the melting point, enthalpy and entropy of fusion, which are obtained via Differential Scanning Calourimetry (DSC) measurements. Vapour pressure data has been obtained for dicarboxylic acids1, substituted diacids2, cyclic diacids3 and substituted benzoic acids4.
References
[1] Booth et al., Atmos. Meas. Tech., 2, 355–361, 2009
[2] Booth et al., Atmos. Chem. Phys. 10, 4879-4892, 2010
[3] Booth et al., Atmos. Chem. Phys. 11, 655-665, 2011
[4] Booth et al., Atmos. Chem. Phys., to be submitted