Structure Activity Relationships (SARs)
In order to understand the atmospheric fate of gaseous organic pollutants and volatile organic compounds (VOCs) in general, it is necessary to know the rate with which they react with atmospheric oxidants such as ozone. There are many VOCs present in the atmosphere, and the total number of identified species has been estimated at 104-105, whose lifetime in the atmosphere span orders of magnitude.
There is a pressing need to characterize the atmospheric transformation of VOCs in order to develop mechanisms for smog chamber studies and detailed mechanisms such as the Master Chemical Mechanism (MCM) to better understand and militate against their undesirable effects on our environment and climate. Furthermore, understanding the physical basis of reactivity can potentially highlight anomalous rate data which may benefit from further investigation experimentally.
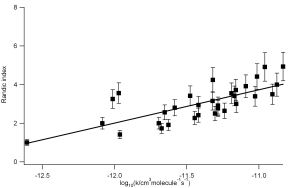
We have used a combination of frontier orbital methods and graph theoretical approach to explain the reactivity of a diverse range of reactions, namely oxidation of VOCs by OH, Cl, NO3 and O3 and radical reactions, see figure 1. As our Structure Activity Relationships have physical basis it is possible to make predictions of rate parameters, including Activation Energy and pre exponential A factors, for related reactions that, as of yet, have not been measured.