Peroxy Radical Kinetics
The study of gas-phase chemical kinetics is central to physical chemistry. In particular, understanding the mechanisms of radical-radical and radical-molecule reactions, such as the factors controlling the pathways for atom recombination, is of fundamental importance. The study of kinetics constitute a central component of research in many areas of chemistry such as organic synthesis, combustion processes, interstellar chemistry, plasma chemistry, chemical vapour deposition, studies of the immune system and the chemistry of the earth's and other planetary atmospheres.
In particular the research is focused on the study of reaction kinetics of importance in urban/regional pollution.
The laboratory data provide the only sound basis for the interpretation of atmospheric behaviour and for the construction of diagnostic and prognostic models. We pursue research in two areas. The first is the development of methods to directly observe the kinetics of radical reactions in the laboratory using chemical ionization mass spectrometry in conjunction with a turbulent flow ‘wall-less’ high-pressure, low temperature flow system (TF-CIMS). The second area of work focuses on the theoretical analysis exploring the patterns in reactivity of radical reactions using Quantitative Structure Activity Relationships (QSARs) and quantum chemical calculations.
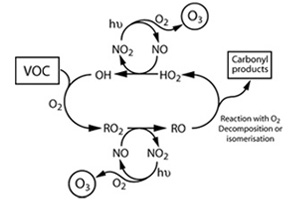
The vital role played by peroxy radicals in the budgets of ozone, NOx, NOy and HOx throughout the troposphere is well documented and the importance of these families in determining atmospheric composition, the earth’s radiative balance and any future change in climate cannot be over-emphasised. However, as our knowledge of the complex interplay between these important families of species has been augmented by field measurements and their comparison with ever more sophisticated computer models, it emerges that certain peroxy radical reactions warrant further examination.
In the polluted troposphere in the presence of NOx, peroxy radicals undergo reaction with NO converting it to NO2 and this ultimately results in ozone production and tropospheric OH.
In the absence of NOx however peroxy radicals can react with HO2 to form hydroperoxides reaction. These can then undergo reaction with OH, be photolysed or get absorbed into cloud droplets and be removed from the troposphere in the form of rain. There is a great need to characterize the peroxy radical reactions to develop mechanisms that are used in atmospheric models of the troposphere.