Scientific Background
An outline of the main scientific issues being addressed by the COBRA project, taken from the COBRA proposal.
Polar bromine release and ozone and mercury depletion events
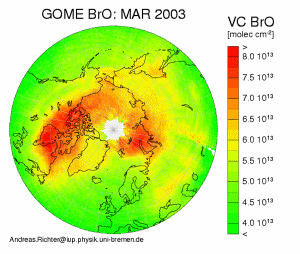
Plot of Northern Hemisphere BrO from the GOME satellite. This plot shows enhanced BrO concentrations over the oceans in the arctic region in spring. Such events last for several weeks, and occur in both hemispheres each year during polar spring. This plot is from the University of Bremen GOME project website at http://www-iup.physik.uni-bremen.de/gome/
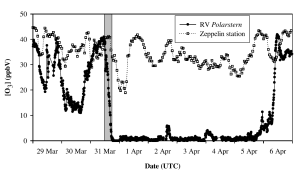
An ozone depletion event measured on board the Polarstern in the Arctic during spring 2003 in the vicinity of new ice fields. Data from a land station at Ny-Alesund, Spitsbergen is shown in grey for comparison, illustrating that the observed ozone depletion is occurring locally rather than being due to long range transport of ozone free air. The grey bar indicates a drop in ozone from 40ppb to close to zero in approximately 7 hours. This plot is from Jacobi et al. 2006 (see references for full citation)
The key role played by bromine in ozone and mercury depletion events has been described in numerous studies (Bottenheim et al., 1986; Schroeder et al., 1998; Bottenheim et al., 2002; Spicer et al., 2002). The suggested autocatalytic heterogeneous mechanism involves uptake of HOBr into slightly acidic and concentrated brines (containing enhanced Br- and Cl-) on sea-ice/snow-pack/sea-salt aerosol surfaces resulting in release of gaseous Br2 and BrCl, which are rapidly photolysed to bromine atoms. The aqueous phase reaction is as follows:
HOBr + X- + H+ --> BrX + H2O, where X = Br, Cl.
Every HOBr molecule entering the liquid phase has the potential to release two bromine atoms to the gaseous phase, resulting in an exponential increase of Br, the so-called "bromine explosion". The resulting photolysable BrX molecules rapidly produce Br atoms that enter various ozone-destroying cycles:
Cycle I
2Br + 2O3 --> 2BrO + 2O2
BrO + BrO --> 2Br + O2
Cycle II
Br+O3 --> BrO + O2
BrO + HO2 --> HOBr + O2
HOBr + hv --> Br + OH
OH + CO (or VOC) --> HO2 + CO2 (or VOC products)
Cycle I will dominate at high BrO levels, and cycle II at low BrO levels. The re-formation of soluble HOBr(g) in Cycle II, which re-enters the aqueous phase either through uptake on sea-salt aerosol or direct deposition to the snow-pack/sea-ice, results in an autocatalytic mechanism.
Schroeder et al. (1998) first observed the rapid decrease in Hg atoms, now referred to as "MDEs" (mercury depletion events) in the Arctic. The MDEs are accompanied by increases in oxidised, but so far unspeciated, mercury in the snowpack, potentially leading to bioaccumulation of soluble and toxic forms of mercury. Recent measurements of the rate coefficients for reactions of a number of halogen gases with Hg suggested that Br atoms could be a major reactant with Hg in the polar atmosphere (Ariya et al., 2002), and subsequent studies have supported this (e.g. Calvert and Lindberg 2004a; Goodsite et al., 2004; Skov et al., 2004). BrO may also be an important reactant, but there are insufficient studies on its rate of reaction with Hg vapour and its concentration in the polar boundary layer. There has been a recent increase in Hg in the Arctic biosphere, and it has been hypothesized that this results primarily from increases in atmospheric oxidant levels and/or reactive bromine rather than from an increase in Hg emissions (OASIS science plan).
It has been suggested that ozone and mercury depletion events apparently operate almost exclusively in polar spring either (a) because only this season has the combination of strong surface inversions (i.e. isolation of boundary layer air from free tropospheric air), sunlight and sea ice (Lehrer et al., 2004), or (b) because the asymmetry of sea ice/frost flower production compared to the amount of sunlight leads to a strong spring maximum in their product (Kaleschke et al., 2005). However, both of the proposed drivers for this seasonality, and the initial source of bromine atoms required to form HOBr and thus initiate the chain destruction of ozone, are controversial.
Potential role of frost flowers and other surfaces for halogen release

There is substantial evidence that sea salt, either on the sea-ice or snow-pack surface or contained in aerosol, is almost exclusively the source of bromine liberation in polar regions. The high salinity and surface area of frost flowers, which are ice crystals that grow on newly forming sea ice, along with their widespread occurrence as part of the normal process of new sea ice formation, has recently led to proposals that they may be an important part of this process (e.g. Rankin et al., 2002). From an analysis of satellite BrO data and sea ice coverage, Kaleschke et al. (2004) showed that areas potentially covered in frost flowers (FF), i.e. with cold temperatures and open leads, are plausibly the dominant source of bromine in both the Arctic and Antarctic. The exact nature of emissions from frost flowers is not well established and is not yet explicitly treated in global models, and so far there are no in situ field observational studies to confirm or otherwise the important role of FF in bromine release. It is vital that field data is obtained on the nature and magnitude of emissions of halogens as a function of age and type (air/sea-ice differential temperature) of frost-flowers and other sea-ice surfaces, in order to predict the spatial and temporal variability of halogen release, and how this might change in the future.
Brines and frost flowers themselves may be responsible for a significant fraction of sea salt aerosol concentrations over the polar regions (Rankin et al., 2002). However, under typical Arctic conditions, even a flat sea-ice surface (i.e., no frost flowers) provides a factor of ~ 1000 higher surface area than sea salt aerosol (Lehrer et al., 2004). The latter modelling study of Lehrer et al. (2004), although highly parameterised, found that sea salt aerosol alone was not sufficient to sustain the elevated levels of gas phase bromine observed in polar spring, although is an efficient recycler of more stable bromine species such as HBr. However, the study of Evans et al. (2003) was able to adequately simulate the chemistry occurring within an ODE through the use of aerosols alone. Thus there are competing hypotheses for the relative role of the surface and aerosol phase in the production and recycling of bromine. The full suite of analytical tools we will deploy during COBRA will allow these hypotheses to be tested more thoroughly than previous studies have allowed.
Potential sources and role of iodine in the Arctic
Little is known about the role and sources of iodine in polar boundary layer chemistry. Iodine-containing aerosol has been associated with ozone depletion at polar sunrise but also appears in autumn. A well-known and obvious source of iodine compounds are under-ice diatoms, which grow there particularly well because of the benevolent light conditions through less than 1 m of ice, compared to those of open water where they are mixed downward to depths of tens of metres. Many diatoms synthesise methyl iodide and other organic iodine compounds, and the chlorophyll they produce is observable from space in large quantities in open water in the sea ice zone in early summer. However, recent work has suggested abiotic/photochemical sources of iodine in the sea ice zone. In a 10-month campaign at Halley station, Antarctica, the iodine oxide radical, IO, was observed using a boundary layer DOAS (Differential Optical Absorption Spectrometer) (A. Saiz-Lopez and J. M.C. Plane, part of the COBRA team, unpublished data). A clear seasonal cycle was observed: IO was below the detection limit (~1.5 ppt) during polar night, and reached levels of several ppt during spring and summer. Preliminary modelling indicates that the source of the iodine during summer is likely to have been emission from the snowpack, arising from photochemical or thermal processes, rather than directly from the ocean.
Further evidence of iodine in the polar atmosphere comes from recent observations of reactive organoiodines including di-iodomethane (CH2I2) during spring at Kuujjuarapik on the south-east shore of Hudson Bay, at the highest concentrations ever observed in air and associated with trajectories across the frozen Bay with no observable leads (L. J. Carpenter et al., submitted to ES&T, 2005). The absence of local ice leads coupled with the extremely short atmospheric lifetime of CH2I2 indicated that production was from the surface of the sea-ice/snowpack over Hudson Bay. We proposed an abiotic mechanism for the production of polyhalogenated iodo- and bromocarbons, via reaction of HOI and/or HOBr with organic material on the quasi-liquid layer above sea-ice/snow pack, and reported laboratory data to support this mechanism. The presence of this mechanism in the natural world (as opposed to in the lab) is at present entirely speculative, however, it might explain the so far puzzling enrichment in CHBr3 observed in upper sea-ice (enhanced in salinity but low in chlorophyll) of the Canadian Arctic (Sturges et al., 1997). It also suggests the possibility for photochemical inorganic iodine release from sea-ice, snow-pack and/or FF, analogous to that recently observed by Zingler and Platt (2005) over the Dead Sea, and consistent with the Antarctic IO observations of Saiz-Lopez and J. M.C. Plane described above. Photochemical reactions in the snow are known to release a wide range of trace gases to the polar atmosphere (e.g. Sumner and Shepson., 1999). Photochemistry within sea-ice is also perfectly feasible, with ice potentially more transparent to the UV than to visible radiation (Belzile et al., 2000), yet has not been well studied. In sea-ice, fresh-water ice co-exists with solid salts, concentrated brines, gases and marine-derived organic material, thus starting material is present for both organic and inorganic halogen release.
These recent and so far unpublished IO and organoiodine observations of the COBRA team, in separate polar locations, suggest a widespread and likely abiotic/photochemical source of iodine to the polar atmosphere. The impact of such emissions may be significant. Theoretical studies indicate that iodine compounds added to a Br2/BrCl mixture in the Arctic atmosphere have a significantly greater ozone and mercury depletion effect than additional bromine molecules (Calvert and Lindberg, 2004a and 2004b).& The role, if any, of iodine compounds on Hg-atoms is unknown. Direct reactions of I atoms with Hg may be unimportant (Goodsite et al., 2004), however a recent study by Calvert and Lindberg (2004b) points out a potential indirect effect of iodine through an enhancement in [Br] mediated by IO and BrO interactions. In mid-latitude coastal locations, release of iodine is known to result in new particle formation and there is a possibility that similar mechanisms operate in the Arctic.
Potential for future change in Arctic halogen-related processes
The natural phenomena described above are likely to be affected by man-made changes such as increased UV radiation reaching the surface due to stratospheric ozone depletion, acidic deposition affecting pH-dependent halogen activation processes, and changes in the production rate of new sea ice, altering the locations and extent of frost flowers and brines. There are already indications that Arctic ozone depletion events and associated deposition of Hg have increased in recent decades (Lindberg et al., 2002; Tarasick and Bottenheim, 2002; Hollwedel et al., 2004). Possible feedbacks such as these must be investigated in order to understand the effects of global change.