Mobile Chemical Ionisation Mass Spectrometer

Two mobile Chemical Ionisation Mass Spectrometers (CIMS) are available for measurements of trace gases in the field and laboratory. The CIMS instruments employed here were built by the Georgia Institute of Technology as previously described by Nowak et al. (2007).
Chemical Ionisation is a very versatile method to produce charged molecules (ions) that can be detected using a mass spectrometer. It is a soft ionisation technique that produces little ion fragmentation and the resultant mass spectra are simplified (compared to electron impact ionisation for instance). Both positively and negatively charged ions can be produced and detected, and with the use of different ionisation schemes (i.e. different reagent ions) CIMS is a highly sensitive and selective method to detect trace gases in the atmosphere.
Ionisation generally occurs at pressures of ~20 Torr in the Ion-Molecule Region (IMR):
A+ + B ![]() B+ + A
B+ + A
where A+ is the reagent ion that specifically has the potential to ionise certain molecules, B is the molecule that is to be detected and which in the process of collision with the reagent ion A+ becomes ionised by charge transfer or by forming an adduct with the reagent ion.
The ionisation schemes currently used here are listed in the table.
Ionisation scheme |
Ion mode |
Gases detected |
Protonated acetone dimer |
Positive |
NH3 |
Iodine I- |
Negative |
HNO3, NO3, HCN, CH2O2, C2H4O2, C3H6O2, C4H8O2 |
Sulfur hexafluoride SF6- |
Negative |
Cl, Cl2, ClO, Cl2O2, Cl2O |
Ammonia (NH3)
The mobile CIMS has been used for measurements of ammonia (NH3) concentrations and fluxes in the field experiments as part of the NitroEurope instrument comparison campaign where measurements were made over a Scottish fertilised grassland (von Bobrutzki, 2010)
Halogens
The mobile CIMS is also used for reaction kinetics studies, measuring halogen compounds relevant to stratospheric photochemistry. Recently work has been carried out in collaboration with The University of Birmingham
Inorganic and organic acids
One of the main research foci at
Manchester is the study of organic acids using CIMS.
The CIMS is used to measure nitric acid (HNO3), hydrogen cyanide (HCN) and organic acids, such as formic acid (CH2O2), acetic acid (C2H4O2), propanoic acid (C3H6O2) and butanoic acid (C4H8O2).
Organic acids are of particular interest because they are ubiquitous in the gas and aerosol phase and they are common constituents of global precipitation. The contribution of organic acids to the acidity of precipitation and subsequent effects on aquatic and terrestrial ecosystems is well known. Formic (HCOOH) and acetic acid (CH3COOH) can dominate free acidity of precipitation thereby having an influence on pH-dependent chemical reactions and OH cloud chemistry. Low molecular weight organic salts are also present in the fine fraction of aerosols, whose physical properties, namely hygroscopicity, include relatively low critical supersaturations, allowing for the activation of cloud droplets and subsequently affecting the total indirect radiative forcing. The sources of organic acids are manifold, they are partly directly emitted (from both biogenic and anthropogenic sources) and they are also partly produced by oxidation of hydrocarbons in the atmosphere. The knowledge on the atmospheric organic acid budgets is limited and an active research area. Collaboration is with University of Bristol
Mobile CIMS in the laboratory
Formic acid (CH2O2) concentrations are measured as part of gas phase chemical reaction product studies, investigating the product yields of formic acid from ethene and isoprene ozonolysis. For this, EXTRA is used as the reaction chamber from which the CIMS samples.
Mobile CIMS on the Atmospheric Research Aircraft
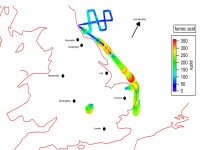
The CIMS has been used on the FAAM Bae-146 aircraft as part of the RONOCO project. The CIMS has successfully deployed for airborne measurements of ammonia, nitric acid and organic acids such as e.g. formic acid, as shown in the figure 4 below.
References
von Bobrutzki et al., 2010. Field inter-comparison of eleven atmospheric ammonia measurement techniques. Atmospheric Measurement Techniques, 3, 91-112.

